What Will Be The Effect Upon The Equilibrium If The Pressure On The System Is Increased
Le Chatelier'south Principle Fundamentals
- Page ID
- 96656
Le Cha telier'south principle states that if a dynamic equilibrium is disturbed by irresolute the weather condition, the position of equilibrium shifts to counteract the change to reestablish an equilibrium. If a chemic reaction is at equilibrium and experiences a change in pressure, temperature, or concentration of products or reactants, the equilibrium shifts in the opposite management to offset the change. This page covers changes to the position of equilibrium due to such changes and discusses briefly why catalysts have no effect on the equilibrium position.
Introduction
An action that changes the temperature, pressure level, or concentrations of reactants in a system at equilibrium stimulates a response that partially offsets the alter while a new equilibrium condition is established (2). Hence, Le Châtelier's principle states that any alter to a organisation at equilibrium will arrange to compensate for that change. In 1884 the French chemist and engineer Henry-Louis Le Châtelier proposed one of the fundamental concepts of chemical equilibria, which describes what happens to a system when something briefly removes information technology from a state of equilibrium.
It is of import to understand that Le Châtelier's principle is merely a useful guide to place what happens when the conditions are changed in a reaction in dynamic equilibrium; it does not give reasons for the changes at the molecular level (due east.g., timescale of alter and underlying reaction machinery).
Concentration Changes
Le Châtelier'southward principle states that if the system is changed in a way that increases the concentration of ane of the reacting species, information technology must favor the reaction in which that species is consumed. In other words, if in that location is an increase in products, the reaction caliber, \(Q_c\), is increased, making it greater than the equilibrium constant, \(K_c\). Consider an equilibrium established betwixt four substances, \(A\), \(B\), \(C\), and \(D\):
\[ A + 2B \rightleftharpoons C + D\]
Increasing a concentration
What happens if conditions are altered by increasing the concentration of A?
Co-ordinate to Le Châtelier, the position of equilibrium will move in such a style equally to counteract the change. In this case, the equilibrium position will move so that the concentration of A decreases again by reacting it with B to grade more C and D. The equilibrium moves to the right (indicated by the green arrow below).

In a practical sense, this is a useful way of converting the maximum possible amount of B into C and D; this is advantageous if, for example, B is a relatively expensive material whereas A is inexpensive and plentiful.
Decreasing a concentration
In the opposite example in which the concentration of A is decreased, co-ordinate to Le Châtelier , the position of equilibrium will motility and so that the concentration of A increases once more. More than C and D will react to supplant the A that has been removed. The position of equilibrium moves to the left.

This is substantially what happens if ane of the products is removed as presently as it is formed. If, for instance, C is removed in this way, the position of equilibrium would movement to the right to replace it. If it is continually removed, the equilibrium position shifts further and farther to the right, effectively creating a ane-fashion, irreversible reaction.
Pressure Changes
This but applies to reactions involving gases, although non necessarily all species in the reaction need to be in the gas stage. A general homogeneous gaseous reaction is given below:
\[ A(g) + 2B(m) \rightleftharpoons C(g) + D(g)\]
Increasing the pressure level
According to Le Châtelier, if the pressure is increased, the position of equilibrium will move so that the pressure is reduced again. Pressure is caused by gas molecules hitting the sides of their container. The more than molecules in the container, the college the pressure will be. The organization can reduce the pressure by reacting in such a way as to produce fewer molecules.

In this example, there are 3 moles on the left-paw side of the equation, but but two on the right. By forming more than C and D, the system causes the pressure to reduce. Increasing the force per unit area on a gas reaction shifts the position of equilibrium towards the side with fewer moles of gas molecules.
Example 1: Haber Process
\[ N_2 + 3H_2 \rightleftharpoons 2NH_3 \]
If this mixture is transferred from a i.five 50 flask to a 5 L flask, in which direction does a internet change occur to render to equilibrium?
Solution
Because the volume is increased (and therefore the force per unit area reduced), the shift occurs in the direction that produces more moles of gas. To restore equilibrium the shift needs to occur to the left, in the direction of the opposite reaction.
Decreasing the force per unit area
The equilibrium will movement in such a way that the pressure increases over again. It can do that past producing more than gaseous molecules. In this example, the position of equilibrium will motility towards the left-hand side of the reaction.

What happens if at that place are the aforementioned number of molecules on both sides of the equilibrium reaction?
In this case, increasing the pressure has no effect on the position of the equilibrium. Because there are equal numbers of molecules on both sides, the equilibrium cannot movement in any way that will reduce the pressure over again. Once more, this is not a rigorous caption of why the position of equilibrium moves in the ways described. A mathematical treatment of the explanation tin can exist institute on this folio.
Summary of Force per unit area Effects
Three ways to change the pressure of an equilibrium mixture are: 1. Add or remove a gaseous reactant or product, 2. Add together an inert gas to the constant-book reaction mixture, or three. Change the volume of the system.
- Adding products makes \(Q_c\) greater than \(K_c\). This creates a net change in the reverse direction, toward reactants. The opposite occurs when adding more reactants.
- Adding an inert gas into a gas-phase equilibrium at constant volume does not result in a shift. This is because the improver of a non-reactive gas does non change the partial pressures of the other gases in the container. While the total pressure of the organization increases, the total pressure does not accept any consequence on the equilibrium constant.
- When the volume of a mixture is reduced, a net change occurs in the direction that produces fewer moles of gas. When book is increased the alter occurs in the direction that produces more moles of gas.
Temperature Changes
To understand how temperature changes affect equilibrium conditions, the sign of the reaction enthalpy must be known. Assume that the frontwards reaction is exothermic (rut is evolved):
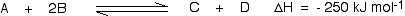
In this reaction, 250 kJ is evolved (indicated by the negative sign) when i mole of A reacts completely with 2 moles of B. For reversible reactions, the enthalpy value is always given as if the reaction was one-way in the frontward direction. The back reaction (the conversion of C and D into A and B) would be endothermic, absorbing the aforementioned corporeality of oestrus.
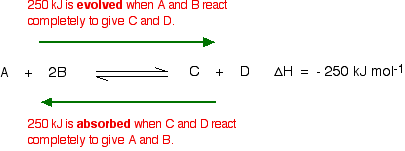
The main effect of temperature on equilibrium is in changing the value of the equilibrium constant.
Temperature is Neither a Reactant nor Product
Information technology is non uncommon that textbooks and instructors to consider oestrus as a independent "species" in a reaction. While this is rigorously wrong because i cannot "add or remove heat" to a reaction as with species, it serves as a convenient machinery to predict the shift of reactions with changing temperature. For example, if heat is a "reactant" (\(\Delta{H} > 0 \)), and so the reaction favors the formation of products at elevated temperature. Similarly, if heat is a "product" (\(\Delta{H} < 0 \)), so the reaction favors the formation of reactants. A more authentic, and hence preferred, clarification is discussed beneath.
Increasing the temperature
If the temperature is increased, and so the position of equilibrium will motility so that the temperature is reduced again. Suppose the organisation is in equilibrium at 300°C, and the temperature is increased 500°C. To cool down, it needs to absorb the extra heat added. In the case, the back reaction is that in which estrus is captivated. The position of equilibrium therefore moves to the left. The new equilibrium mixture contains more A and B, and less C and D.
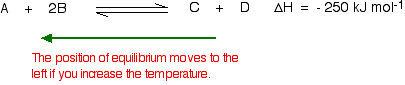
If the goal is to maximize the amounts of C and D formed, increasing the temperature on a reversible reaction in which the forrard reaction is exothermic is a poor arroyo.
Decreasing the temperature?
The equilibrium volition move in such a way that the temperature increases once more. Suppose the system is in equilibrium at 500°C and the temperature is reduced to 400°C. The reaction will tend to heat itself upward over again to return to the original temperature by favoring the exothermic reaction. The position of equilibrium will move to the right with more than \(A\) and \(B\) converted into \(C\) and \(D\) at the lower temperature:

Example two
Consider the germination of water
\[O_2 + 2H_2 \rightleftharpoons 2H_2O\;\;\; \Delta{H}= -125.seven\, kJ\]
- What side of the reaction is favored? Considering the heat is a product of the reaction, the reactants are favored.
- Would the conversion of \(O_2\) and \(H_2\) to \(H_2O\) exist favored with heat as a product or as a reactant? Oestrus every bit a product would shift the reaction forward, creating \(H_2O\). The more heat added to the reaction, the more \(H_2O\) created
Summary of Temperature Effects
- Increasing the temperature of a organisation in dynamic equilibrium favors the endothermic reaction. The system counteracts the change by absorbing the extra rut.
- Decreasing the temperature of a system in dynamic equilibrium favors the exothermic reaction. The system counteracts the change by producing more than heat.
Catalysts
Calculation a catalyst makes absolutely no divergence to the position of equilibrium, and Le Châtelier'south principle does not apply. This is because a catalyst speeds up the forrard and back reaction to the same extent and adding a catalyst does not affect the relative rates of the 2 reactions, it cannot touch the position of equilibrium.
Even so, catalysts accept some awarding to equilibrium systems. For a dynamic equilibrium to be set upwards, the rates of the forrad reaction and the dorsum reaction must be equal. This does not happen instantly and for very boring reactions, it may take years! A catalyst speeds up the rate at which a reaction reaches dynamic equilibrium.
Example 3
You might try imagining how long it would take to establish a dynamic equilibrium if you took the visual model on the introductory page and reduced the chances of the colors changing by a factor of chiliad - from 3 in half dozen to 3 in 6000 and from 1 in vi to 1 in 6000. Starting with blue squares, by the stop of the time taken for the examples on that folio, you would most probably still have entirely blue squares. Eventually, though, you would finish up with the same sort of patterns every bit before - containing 25% blueish and 75% orange squares.
Problems
1. Varying Concentration
What will happen to the equilibrium when more 2SO2 (g) is added to the following system?
\[2SO_2(g) + O_2(g) \rightleftharpoons 2SO_3 (g) \]
Solution:
Adding more reactants shifts the equilibrium in the direction of the products; therefore, the equilibrium shifts to the right.
Overall, the concentration of \(2SO_2\) from initial equilibrium to terminal equilibrium volition increase because only a portion of the added amount of \(2SO_2\) will be consumed.
The concentration of \(O_2\) volition decrease because as the equilibrium is reestablished, \(O_2\) is consumed with the \(2SO_2\) to create more \(2SO_3\). The concentration of \(2SO_3\) will be greater because none of it is lost and more is being generated.
2. Varying Pressure
What volition happen to the equilibrium when the volume of the organisation is decreased?
\[2SO_{2(m)} + O_{2 (chiliad)} \rightleftharpoons 2SO_{3 (g)}\]
Solution:
Decreasing the volume leads to an increment in pressure which volition crusade the equilibrium to shift towards the side with fewer moles. In this instance there are iii moles on the reactant side and ii moles on the production side, and so the new equilibrium will shift towards the products (to the right).
3. Varying Temperature
What volition happen to the equilibrium when the temperature of the arrangement is decreased?
\[N_{ii(g)} + O_{2 (thou)} \rightleftharpoons 2NO_{(one thousand)} \;\;\;\; \Delta{H} = 180.v\; kJ\]
Solution
Because \(\Delta{H}\) is positive, the reaction is endothermic in the forwards management. Removing heat from the arrangement forces the equilibrium to shift towards the exothermic reaction, so the reverse reaction will occur and more reactants will be produced.
References
- Pauling, L., Higher Chemistry, 3rd ed., Freeman, San Francisco, CA, 1964.
- Petrucci, R., Harwood, W., Herring, F., Madura, J., General Chemistry, 9th ed., Pearson, New Jersey, 1993.
- www.jce.divched.org/Journal/I...2N08/p1190.pdf
- Huddle, Benjamin P. "Conceptual Questions" on LeChatelier's Principle." J. Chem. Educ. 1998 75 1175.
- Thomsen, Volker B. E. " Le Chatelier's Principle in the Sciences." J. Chem. Educ. 2000 77 173.
Contributors and Attributions
- Amanda Wolf (UCD)
-
Jim Clark (Chemguide.co.uk)
What Will Be The Effect Upon The Equilibrium If The Pressure On The System Is Increased,
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Le_Chateliers_Principle/Le_Chatelier's_Principle_Fundamentals
Posted by: williamssaver1959.blogspot.com


0 Response to "What Will Be The Effect Upon The Equilibrium If The Pressure On The System Is Increased"
Post a Comment